CS1232 and Sulfur by Infrared
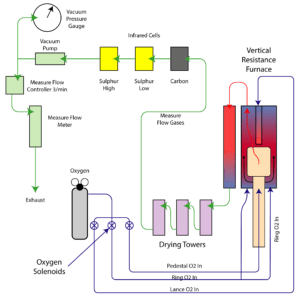
The CS1232 Sulfur analyser reads organic Sulfur by converting the S to SO2. This conversion process takes place when the organic material, usually pulverised to 70 um or less, is placed into a crucible and then into the combustion furnace. The furnace is purged of all interfering atmosphere with Oxygen to provide an Oxygen rich enviroment. When the sample begins to combust, the Sulfur atoms, S, combines with the Oxygen atoms, O2, and forms SO2. In the Infrared spectrum, the resulting signal of the SO2 is “absorbed” and a loss in signal is observed. This loss of signal is then inverted into a positive plot onscreen for the operator to witness.
Once the infrared signal begins to normalise back to the starting voltage (baseline), the Sulfur analyser then computes if the sample is finished when no significant SO2 is observed. The CS1232 then outputs the result as a percentage of CO2.
In all cases, the combusted sample is drawn through a moiture trap to remove any residual moisture that may be present in the sample. In the case of Sulfur this step is critical to remove any interferences between the SO2 and H2O bands in the infrared spectrum. All manufacturers of Sulfur analysers utilising infrared detection must do this. The sample is then pulled through the IR detection system at a controlled rate set by the flow controller and pushed through the measure flow meter and then out to atmosphere.
The CS1232 Sulfur analyser only reads SO2. This is set to the specific infrared waveband filter that blocks all IR light in the spectrum except that of SO2