Theory of Operation
The CS1232 Carbon Sulfur Analyzer Principle is a non-dispersive, infrared, PC controlled instrument designed to measure the Carbon and Sulfur content in wide variety of organic materials including, Coal, Coke, Oil, as well as some inorganic materials including, Soil, Mineral Ores, Cement and Limestone to name a few.
The analysis begins by weighing a sample (0.350 gm nominal) into the combustion crucible. The sample is then placed into the combustion furnace, which is regulated at 1350°C, within an oxygen rich environment. The combination of the furnace temperature and the oxygen causes the sample to combust.
All sample materials contained in the combustion crucible go through an oxidative reduction process that causes the Carbon and Sulfur bearing compounds to break down freeing the Carbon and Sulfur. The Carbon oxidizes to form CO2 and the Sulfur oxidizes to form SO2. The design of the combustion system prevents atmosphere from entering the sample combustion zone.
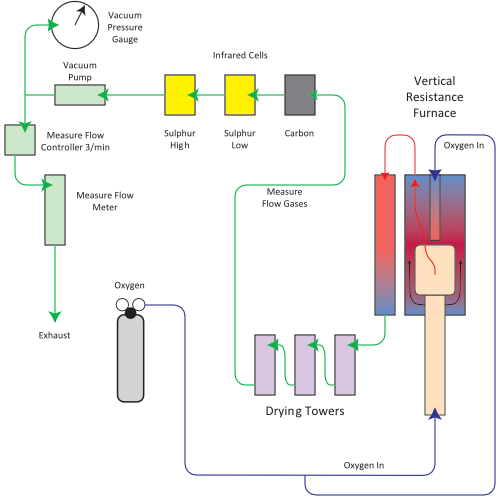
After a preset time, the oxygen begins to flow through the lance to accelerate the combustion of hard to combust materials and the Carbon and Sulfur (as CO2 and SO2) is released into the measure flow as sample gases.
Sample gases are first swept through the furnace top plate to be collected by the collection tube, effectively allowing the sample gases to remain in the high temperature zone, permitting efficient oxidation.
From the combustion system, the gases pass through two Magnesium Perchlorate (MgClO4) drying towers to remove moisture and a particulate filter to remove any particulates from the sample gases. The gases then pass through a flow controller, which controls the measure flow at 3l/min, then finally through the infrared detection cell.
The Carbon and Sulfur infrared cell/s measures the concentration of the Sulfur dioxide and the Carbon dioxide present in the sample. The instrument converts these signal values to a percentage value, using an equation preset in the software taking into account the sample weight, calibration, and peak of the sample curve and area of the combusted sample.
Infrared Radiation, Absorption and Detection
All molecules, with the exception of dipolar species such as N2, H2 and O2, absorb in the infrared region. Because no two compounds absorb the same way, infrared spectrometry is an extremely useful method for qualitative and quantitative chemical analysis. As radiant energy is projected through the sample gases, an infrared absorption spectrum is produced. Since all molecules have a characteristic spectrum, the identity and quality of a compound can be determined.
In the infrared detector cell, energy is emitted from an infrared source. Radiant energy, at a frequency of 8Hz, enters the infrared cell through a calcium fluoride lens, and is projected through the sample gases in the cell chamber. Gases absorb the infrared energy producing a spectrum. As the energy passes through the second calcium fluoride lens and exits the cell chamber, a detector fitted with a precise wavelength filter, selectively blocks all wavelengths except that of the CO2 and SO2.
The detector then responds to the energy changes between the carrier gas and sample gas, to determine the Carbon and Sulfur concentration of the sample.
The detector output is coupled to an electronic circuit where the signal is amplified, rectified and filtered. The analogue voltage is then converted to a digital signal by an analogue to digital converter card in the PC. The nominal voltage of the infrared cells is between 5 and 6.0 VDC.
Prior to the start of an analysis, the computer establishes the baseline. As the analysis begins the infrared cell output voltage decreases proportionally with the amount of Sulfur and Carbon present in the infrared cell. The computer reads this output several times a second and is converted to an equivalent mass per second by an interval linearization equation. The result is corrected by calibration, sample weight, the height of the sample peak and the sample area of the combusted sample.